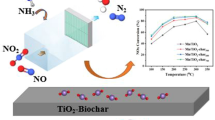
Abstract
Copper oxide supported on a biochar could suffer from a low nitrogen-selectivity and carbon combustion, despite the sustainability prospect it provides in replacing the utilization of non-renewable materials as the catalyst support to reduce nitric oxide. Bimetallic catalysis is a means to improve catalytic properties including conversion and selectivity. Therefore, this work investigates the enhancement of carbon-supported copper oxides in nitric oxide selective reduction using hydrogen by co-impregnating iron or manganese with copper. The bimetallic catalysts were prepared via sequential incipient wetness method where copper oxide was impregnated and calcined prior to the impregnation and calcination of the co-catalyst. As iron was paired with copper, the nitrogen selectivity was enhanced by 20% (almost 100% selective) at 200 °C while reducing the carbon combustion rate by 20% at a higher temperature (300 °C). This improvement was regarded as the synergistic effects obtained by the bimetallic oxide catalysts as a result of the altered elemental composition (from 60% carbon content to 50%), catalyst acidity (from 12 mmol NH3 desorbed/g to 16 mmol NH3d/g) and redox properties (from 5 mmol H2 consumed/g to 3 mmol H2/g). Flash elemental analyser showed that this catalyst has lower carbon content but higher oxygen amount (30% compared to 19%) which is correlated to the higher acidic sites, as confirmed via temperature-programmed desorption analysis and Fourier-Transform infra-red spectroscopy. Varying the ratio between the two catalysts revealed that different mechanisms govern the reaction that could be the key to understanding the enhancement of the nitrogen-selectivity. Nevertheless, further studies are required to ensure the applicability of this catalytic system in the industry.













Similar content being viewed by others
Notes
The latter phenomenon was proven to occur by heating the used catalysts after reaction to a higher temperature in pure helium flow and measuring the desorbed species with MS. The desorption profile for an example is included in Supporting Information Fig. A4.
References
Aldhalmi AK, Alkhayyat S, Younis Albahadly WK, Jawad MA, Alsaraf KM, Riyad Muedii ZA-H, Ali FA, Ahmed M, Asiri M, Al-Fatolahi L, Fakhri A (2023) A novel fabricate of iron and nickel-introduced bimetallic MOFs for quickly catalytic degradation via the peroxymonosulfate, antibacterial efficiency, and cytotoxicity assay. Inorg Chem Commun 153:110823. https://doi.org/10.1016/j.inoche.2023.110823
Ali M, Hussain I, Mehmud I, Umair M, Hu S, Muhammad H, Sharif A (2021) Recent breakthroughs and advancements in NOx and SOx reduction using nanomaterials-based technologies: A state-of-the-art review. Nanomater 11:1–20. https://doi.org/10.3390/nano11123301
Allen AE, Macmillan DWC (2012) Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem Sci 2012:633–658. https://doi.org/10.1039/C2SC00907B
Bahadoran A, Liu Q, Liu B, Gu J, Zhang D, Fakhri A (2021) Fabrication and structural of gold / cerium nanoparticles on tin disulfide nanostructures and decorated on hyperbranched polyethyleneimine for photocatalysis, reduction, hydrogen production and antifungal activities. J Photochem Photobio A: Chem 416(April):113316. https://doi.org/10.1016/j.jphotochem.2021.113316
Blanco H, Lima SH, Rodrigues VDO, Palacio LA, Faro C (2019) Copper-manganese catalysts with high activity for methanol synthesis. Appl Catal A: Gen 579:65–74. https://doi.org/10.1016/j.apcata.2019.04.021
Burch R, Coleman MD (1999) An investigation of the NO/H2/O2 reaction on noble-metal catalysts at low temperatures under lean-burn conditions. Appl Catal B: Environ 23:115–121. https://doi.org/10.1016/S0926-3373(99)00073-9
Camposeco R, Castillo S, Mugica V, Mejía-Centeno I, Marín J (2014) Role of V2O5-WO3/H2Ti3O7-nanotube-model catalysts in the enhancement of the catalytic activity for the SCR-NH3 process. Chem Eng J 242:313–320. https://doi.org/10.1016/j.cej.2014.01.002
Chen Y, Jihad A, Hussam F, Al-Abdeen SHZ, Hussein JM, Adhab ZH, Alzahraa ZHA, Ahmad I, Fatolahi L, Janani BJ (2023) A facile preparation method for efficiency a novel LaNiO3/SrCeO3 (p-n type) heterojunction catalyst in photocatalytic activities, bactericidal assessment and dopamine detection. Surfaces Interfaces 38:102830. https://doi.org/10.1016/j.surfin.2023.102830
Chin TY, Yakub I, Yun Hin T-Y (2020) Evaluation of catalysts derived from palm kernel shell carbon in a passive NOx removal from a diesel engine exhaust. Emiss Control Sci and Technol 6(3):336–344. https://doi.org/10.1007/s40825-020-00164-0
de Nevers N (1995) Air pollution control engineering. McGraw-Hill
da Luz Corrêa AP, da Silva PMM, Gonçalves MA, Bastos RRC, da Rocha Filho GN, da Conceição LRV (2023) Study of the activity and stability of sulfonated carbon catalyst from agroindustrial waste in biodiesel production: Influence of pyrolysis temperature on functionalization. Arabian J Chem 16(8):104964. https://doi.org/10.1016/j.arabjc.2023.104964
Doan T, Dam P, Nguyen K, Vuong TH, Le MT, Pham TH (2020) Copper-Iron bimetal ion-exchanged SAPO-34 for NH3-SCR of NOx. Catal 10:1–20. https://doi.org/10.3390/catal10030321
European Commission (2006) Reference document on the best available techniques for waste incineration. In: Integr Pollut Prev Control (Issue August). https://doi.org/10.1002/0470012668.ch5
Fang D, Xie J, Hu H, Yang H, He F, Fu Z (2015) Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem Eng J 271:23–30. https://doi.org/10.1016/j.cej.2015.02.072
Gao Y, Gong SY, Chen B, Xing WH, Fei YF, Hu ZT, Pan Z (2022) Progress in metal-organic framework catalysts for selective catalytic reduction of NOx: A mini-review. Atmos 13(5):1–11. https://doi.org/10.3390/atmos13050793
Guan B, Zhan R, Lin H, Huang Z (2014) Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl Therm Eng 66(1–2):395–414. https://doi.org/10.1016/j.applthermaleng.2014.02.021
Hitaishi V, Nandam A (2024) Mid-IR supercontinuum generation using a slab with D-shaped/semi-ellipse core waveguide at 4μm. Opt Mater 147:114668. https://doi.org/10.1016/j.optmat.2023.114668
Holder JV (2002) Chapter 3: Chemistry and the environment. Handbook of Green Chemistry & Technology. Blackwell Science, pp 28–55
Hou Y, Cai G, Huang Z, Han X, Guo S (2014) Effect of HCl on V2O5/AC catalyst for NO reduction by NH3 at low temperatures. Chem Eng J 247:59–65. https://doi.org/10.1016/j.cej.2014.01.036
Iwanek EM, Liotta LF, Williams S, Hu L (2020) Application of potassium ion deposition in determining the impact of support reducibility on catalytic activity of Au/Ceria-Zirconia catalysts in CO oxidation, NO oxidation, and C3H8 combustion. Catal 10:1–13. https://doi.org/10.3390/catal10060688
Japke E, Casapu M, Trouillet V, Deutschmann O, Grunwaldt JD (2015) Soot and hydrocarbon oxidation over vanadia-based SCR catalysts. Catal Today 258:461–469. https://doi.org/10.1016/j.cattod.2015.04.020
Jia X, Peng R, Huang H, Dan J, Lu M, Zhang D, Wang J, Li D, Fang H, Yu C (2022) Lotus leaves-derived MnOx/biochar as an efficient catalyst for low-temperature NH3-SCR removal of NOx: effects of modification methods of biochar. J Chem Tech Biotech 97(11):3100–3110. https://doi.org/10.1002/jctb.7175
Kamsuwan T, Krutpijit C, Praserthdam S, Phatanasri S, Jongsomjit B, Praserthdam P (2021) Comparative study on the effect of different copper loading on catalytic behaviors and activity of Cu/ZnO/Al2O3 catalysts toward CO and CO2 hydrogenation. Heliyon 7(7):e07682. https://doi.org/10.1016/j.heliyon.2021.e07682
Kim HS, Kasipandi S, Kim J, Kang SH, Kim JH, Ryu JH, Bae JW (2020) Current catalyst technology of selective catalytic reduction (SCR) for NOx removal in South Korea. Catal 10(1). https://doi.org/10.3390/catal1001005
Leishman C, Mazzone S, Sun Y, Bekris L, Papaioannou EI, Li K, García-García FR (2023) Manganese-based catalysts supported on carbon xerogels for the selective catalytic reduction of NOx using a hollow fibre-based reactor. Catal Today. https://doi.org/10.1016/j.cattod.2023.01.026
Li Q, Yang H, Ma Z, Zhang X (2012) Selective catalytic reduction of NO with NH3 over CuOx-carbonaceous materials. Catal Commun 17:8–12. https://doi.org/10.1016/j.catcom.2011.10.008
Liu C, Wang H, Bi Y, Zhang Z (2020) A study on the selective catalytic reduction of NOx by ammonia on sulphated iron-based catalysts. RSC Adv 10(67):40948–40959. https://doi.org/10.1039/d0ra06697d
Liu Y, Zong L, Zhang C, Liu W, Fakhri A, Kumar V (2021) Design and structural of Sm-doped SbFeO3 nanopowders and immobilized on poly (ethylene oxide) for efficient photocatalysis and hydrogen generation under visible light irradiation. Surfaces Interfaces 26:101292. https://doi.org/10.1016/j.surfin.2021.101292
Ma Z, Yang H, Li Q, Zheng J, Zhang X (2012) Catalytic reduction of NO by NH3 over Fe-Cu-Ox/CNTs-TiO2 composites at low temperature. Appl Catal A: Gen 427–428:43–48. https://doi.org/10.1016/j.apcata.2012.03.028
Mahdi AA, Obeid RA, Abdullah K, Mohammed S, Kadhim AJ, Ramadan MF, Hussien BM, Alkahtani A, Ali FA, Alkhathami AG, Al-Fatolahi L, Fakhri A (2023) A facile construction of NiV2O6/CeO2 nano-heterojunction for photo-operated process in water remediation reaction, antibacterial studies, and detection of D-Amino acid in peroxidase system. Surfaces Interfaces 40:102970. https://doi.org/10.1016/j.surfin.2023.102970
Mohan S, Dinesha P, Kumar S (2020) NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem Eng J 384:123253. https://doi.org/10.1016/j.cej.2019.123253
Moreno-Piraján JC, Tirano J, Salamanca B, Giraldo L (2010) Activated carbon modified with copper for adsorption of propanethiol. Int J Mol Sci 11(3):927–942. https://doi.org/10.3390/ijms11030927
Muzammil K, Zaid M, Abdul-Reda Hussein U, Abduljabbar MH, Jalal SS, Najm MAA, Alshahrani MY, Almulla AF, Alsaalamy A, Amer RF, Janani BJ (2023) Decoration of MoO3-x on clay mineral matrix with great phosphorescence properties for oxygen activation, photochemical properties, bactericidal and oxidase-like mimics for prompt detection of pesticide. Mater Sci Semiconductor Process 168:107847. https://doi.org/10.1016/j.mssp.2023.107847
Patel A, Rufford TE, Rudolph V, Zhu Z (2011) Selective catalytic reduction of NO by CO over CuO supported on SBA-15: effect of CuO loading on the activity of catalysts. Catal Today 166(1):188–193. https://doi.org/10.1016/j.cattod.2010.05.040
Peng Z, Pan Y, Shen X, Yao L, Bentalib A, Yang J, Zeng J (2019) Competitive transient electrostatic adsorption for in situ regeneration of poisoned catalyst. ChemCatChem 11(4):1179–1184. https://doi.org/10.1002/cctc.201802055
Poreddy R, Mossin S, Jensen AD (2021) Promoting effect of copper loading and mesoporosity on Cu-MOR in the carbonylation of dimethyl ether to methyl acetate. Catal 11(696):1–17. https://doi.org/10.3390/catal11060696
Putrakumar B, Seelam PK, Srinivasarao G, Rajan K, Rajesh R, Rao KR, Liang T (2020) High performance and sustainable copper-modified hydroxyapatite catalysts for catalytic transfer hydrogenation of furfural. Catal 10(9):1045. https://doi.org/10.3390/catal10091045
Savva PG, Costa CN (2011) Hydrogen lean-deNOx as an alternative to the ammonia and hydrocarbon selective catalytic reduction (SCR). Catal Rev 53(2):91–151. https://doi.org/10.1080/01614940.2011.557964
Selvam R, Eshwar D, Shanmugam N, Madraswala A, Babu C, Alphin MS, Sekar M (2022) Biochar supported manganese based catalyst for low-temperature selective catalytic reduction of nitric oxide. Clean Tech Environ Policy 25. https://doi.org/10.1007/s10098-022-02274-5
Semakula M, Inambao PF (2018) The formation, effects and control of oxides of nitrogen in diesel engines. Int J Appl Eng Res 13(6):3200–3209. Available at https://www.ripublication.com/ijaer18/ijaerv13n6_09.pdf. Accessed 22 Oct 2022
Serrano-Lotina A, Cruz K, Bañares MA, Daturi M, Ávila P (2023) Bimetallic MnO2-supported catalysts for selective reduction of NO with NH3 operando IR studies. Appl Surf Sci 610. https://doi.org/10.1016/j.apsusc.2022.155550
Shen Z, Ren S, Zhang B, Bian W, Xing X, Zheng Z (2023) Sulfur and Water Resistance of Carbon-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catal 13(11):1434. https://doi.org/10.3390/catal13111434
Silas K, Ghani WAWAK, Choong TSY, Rashid U (2019) Carbonaceous materials modified catalysts for simultaneous SO2/NOx removal from flue gas: A review. Catal Rev Sci Eng 61(1):134–161. https://doi.org/10.1080/01614940.2018.1482641
Syed A, Elgorban AM, Bahkali AH, Eswaramoorthy R, Verma M, Varma RS, Janani BJ (2023) Highly-impressive performances of novel NiCo2O4/Bi2O3/Ag2ZrO3 nanocomposites in photocatalysis system, removal pathway, toxicity estimation, and antibacterial activities. J Taiwan Inst Chem Eng 149:105004. https://doi.org/10.1016/j.jtice.2023.105004
Tan M, Yakub I, Yun Hin T-Y (2020) Passive nitrogen oxides removal from a diesel-engine exhaust gas using a biomass-carbon catalyst. J Appl Sci Process Eng 7(1):479–488. https://doi.org/10.33736/jaspe.2213.2020
Udayakumar M, Simon A, Katalin A, Hernadi K (2021) Composite carbon foams as an alternative to the conventional biomass-derived activated carbon in catalytic application. Mater 1–11. https://doi.org/10.3390/ma14164540
Wang Y, Li J, Zhang J, Zhang J, Zhai D, Qian G, Liu Y, Zuo C (2019) Advantages of bimetallic nitric oxide reduction catalysts consisting of heavy metals rich in hazardous wastes. J Clean Prod 237. https://doi.org/10.1016/j.jclepro.2019.117834
Wang W, Zhao S, Tang X, Chen C, Yi H (2022) Stainless steel catalyst for air pollution control : structure, properties, and activity. In environmental science and pollution research. Springer: Berlin Heidelberg
Wang J, Sun J, Huang J, Fakhri A, Kumar V (2021) Synthesis and its characterization of silver sulfide/nickel titanate/ chitosan nanocomposites for photocatalysis and water splitting under visible light, and antibacterial studies. Mater Chem Phy 272(July):124990. https://doi.org/10.1016/j.matchemphys.2021.124990
Wei Y, Wang B, Ren R, Wang R (2023) Effects of synthesis conditions on rare earth doped iron oxide catalyst for selective catalytic reduction of NOx with NH3. Aerosol Air Qual Res 23(4):220438. https://doi.org/10.4209/aaqr.220438
WHO (2022) WHO ambient air quality database, 2022 update: status report. In: World health organization vol. 33, issue 1. Available at https://www.who.int/publications/i/item/9789240047693. Accessed 11 June 2023
Xie X, Xie R, Suo Z, Huang H, Xing M, Lei D (2021) A highly dispersed Co–Fe bimetallic catalyst to activate peroxymonosulfate for VOC degradation in a wet scrubber. Environ Sci: Nano 8(10):2976–2987. https://doi.org/10.1039/D1EN00547B
Xu S, Han X, Ma Y, Duong TD, Lin L, Gibson EK, Sheveleva A, Chansai S, Walton A, Ngo DT, Frogley MD, Tang CC, Tuna F, McInnes EJL, Catlow CRA, Hardacre C, Yang S, Schröder M (2021) Catalytic decomposition of NO2 over a copper-decorated metal–organic framework by non-thermal plasma. Cell Rep Phy Sci 2(2):100349. https://doi.org/10.1016/j.xcrp.2021.100349
Yakub I, McGregor J (2021) Catalytic reduction of nitric oxide with hydrogen using carbon-supported d-metal catalysts. Waste Biomass Valor. https://doi.org/10.1007/s12649-021-01623-7
Yao D, Liu B, Wu F, Li Y, Hu X, Jin W, Wang X (2021) N2O formation mechanism during low-temperature NH3-SCR over Cu-SSZ-13 catalysts with different Cu loadings. Ind Eng Chem Res 60(28):10083–10093. https://doi.org/10.1021/acs.iecr.1c01514
Zhang T, Ma S, Chen L, Li R, Leng X, Li Y, Yuan F, Niu X, Zhu Y (2019) Effect of Cu doping on the SCR activity over the CumCe0.1-mTiOx (m = 0.01, 0.02 and 0.03) catalysts. Appl Catal A: Gen 570:251–261. https://doi.org/10.1016/j.apcata.2018.11.025
Zhao T, Feng B, Du J, Shan S, Shi Y (2024) Effects of CO2 on the low-temperature NH3-SCR performance of CeOx-biochar catalyst. Fuel 358:130263. https://doi.org/10.1016/j.fuel.2023.130263
Zumbar T, Arcon I, Djinovic P, Aquilanti G, Gregor Z, Pintar A, Popova M, Logar Z, Ristic A, Draz G (2023) Winning combination of Cu and Fe oxide clusters with an alumina support for low-temperature catalytic oxidation of volatile organic compounds. Appl Mater Interfaces 15:28747–28762. https://doi.org/10.1021/acsami.3c02705
Funding
This work was funded by the Ministry of Higher Education Malaysia under the Fundamental Research Grant Scheme (Grant number: FRGS/1/2020/WAB02/UNIMAS/03/1). The authors wish to thank University Malaysia Sarawak for the support, and James McGregor for his guidance at the Department of Chemical and Biological Engineering, University of Sheffield, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yakub, I., Mohamad Said, K.A., Baini, R. et al. Enhancing the catalytic properties of a biochar-supported copper oxide in nitric oxide selective reduction with hydrogen. Braz. J. Chem. Eng. (2024). https://doi.org/10.1007/s43153-024-00453-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43153-024-00453-z