Esterification of Microwave Induced Pyrolytic Oil from Sago Bark Waste
- 48 Downloads
Abstract
In this study, microwave induced pyrolytic oil from sago bark (SB) was subjected to esterification to improve its properties. Improvement on properties of the pyrolytic oil was performed via esterification with ethanol and the presence of sulfuric acid as a catalyst. The optimum esterification condition was studied using general factorial design. The esterified oil (EO) showed improved properties with pH (4–6), reduced moisture content (2.95–3.45%), density (0.9–1.1 g cm−3), and acid value (39.3–123.4 mg KOH−1 g−1), and maintained the CV (20.2–21.6 MJ kg−1). GC–MS analysis showed that EO was sulphur free, and low in carboxylic acid and oxygenated compounds. The optimal esterification condition with optimum quality of EO was at 65 °C temperature, 60 min reaction time and 1:1 ethanol to oil volume ratio. Results indicated that the EO can be potentially used in robust combustion engines upon properties refinement.
Keywords
Esterification Sago bark Microwave pyrolysis Esterified oilStatement of Novelty
A number of studies have shown that pyrolytic oil upgrading via esterification is well established. However, no comprehensive study was dedicated to upgrading of sago pyrolytic oil via esterification process.
Introduction
Microwave pyrolysis is a promising alternative to resolve the challenges in terms of improving the yield and quality of the resultant liquid biofuels and increase energy efficiency of the whole biomass to liquid fuel product conversion process [1]. However, any pyrolytic oil, more than often has several drawbacks of pyrolytic oil are the low heating value, high viscosity, high corrosiveness and poor stability [2]. The aforementioned problems limit the potential of the pyrolytic oil as fuel material.
Pyrolytic oil is very complex in composition and contains large amount of oxygenated compounds such as aldehydes and ketones [3]. Direct usage of pyrolytic oil for internal combustion is not practical because of the oxygenated compounds contents and high acidity and corrosiveness of the pyrolytic oil. Thus, it is necessary to improve the pyrolytic oil properties by reducing water, oxygenated and acidic content before it can be used as fuel [4].
A number of chemical modification techniques has been studied for upgrading the pyrolytic oil such as hydrodeoxygenation [5], supercritical fluid treatment [6] and esterification [7]. These processes are able to reduce acidity and increase calorific value (CV). In esterification process, the carboxyl groups in the pyrolytic oil are converted to the corresponding esters to improve the quality of pyrolytic oil in terms of odour, corrosiveness and stability [8]. As a result of its simplicity, low temperature and pressure, low cost of some alcohols like methanol, esterification seems to be one of the promising techniques to upgrade the pyrolytic oil [9].
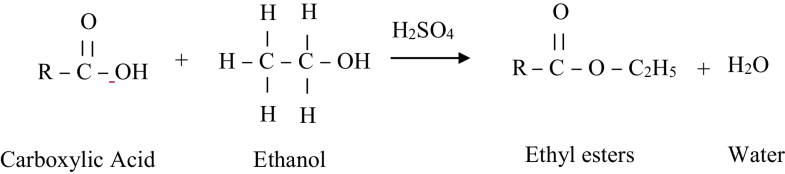
In esterification reaction, carboxylic acids react with alcohol at atmospheric pressure in the presence of an acid catalyst to form alkyl ester. Catalyst is believed to increase the reaction rate and yield [10]. The most common acid catalyst is Bronsted acid such as sulphuric acid [11]. Polar solvent addition during esterification reduces the oil viscosity via the following three mechanisms: (1) physical dilution without affecting the chemical reaction rates; (2) reducing the reaction rate by molecular dilution or by changing the oil microstructure; (3) chemical reactions between the solvent and the oil components that prevent further chain growth [12]. In such case, the reactive molecules of pyrolytic oil like carboxylic acids and aldehydes are converted by the reactions with alcohols to esters and acetals, respectively. Esterification not only decrease viscosity and the aging rate, but also lead to other desirable changes, such as reduced acidity, improved volatility and heating value and better miscibility with diesel fuels [13].
Methanol and ethanol are the most common alcohol used for esterification because of the cost factor [14]. Many biodiesel produced undergo esterification with methanol as it is believed to be more practical than ethanol [15]. Methanol is easy to obtain, and it is able to catalyze reaction at low temperature of the reaction and can achieve high conversion of methyl in minimal time [16]. Ethanol is sometimes preferred over methanol because its higher solubility towards oils [17].
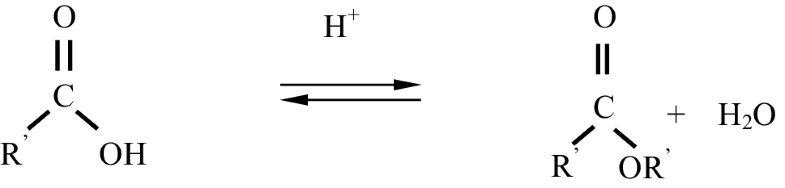
Acid catalyst gives high yields in alkyl esters on a slow reactions to complete the conversion, suitable for waste or unrefined oil. The mechanism of the esterification via acid catalyst of microwave pyrolytic oil of SB is illustrated in Scheme 1. Acid catalyst protonated the C=O forming ester compound and water. The esterification reaction is both slow and reversible. The equation for the reaction between an acid RCOOH and an alcohol R’OH (where R and R’ can be the same or different) is shown in Scheme 1.
Reaction between an acid RCOOH and an alcohol R’OH [13]
In previous studies, esterification was used to remove carboxylic acid in sawdust pyrolytic oil [6] and to improve pH value and CV of palm kernel shell microwave pyrolytic oil employing ethanol as alcohol and sulphuric acid as catalyst [7]. In addition, esterification on babul wood pyrolytic oil by using 1-butanol and cation exchange resin, Amberlyst-15, as a solid acid catalyst successfully reduces the pH of pyrolytic oil [18]. Thus, the present paper reports on the effect of esterification process on the physicochemical properties of SB microwave pyrolytic oil. The physicochemical characteristics of the esterified pyrolytic oil (EO) were assessed, and the suitability of the esterified pyrolytic oil for application as fuel was concluded.
Materials and Methods
SB Microwave Pyrolytic Oil
SB microwave pyrolytic oil used in this study was obtained from microwave pyrolysis of SB in a modified microwave oven with a constant power of 700 W and a frequency of 2.54 GHz (MW71C, Samsung, Japan). The microwave pyrolysis condition was: SB mass (15 g), palm kernel shell activated carbon (35 wt%), temperature (400 °C), and heating time (15 min). The physicochemical properties of SB microwave pyrolytic oil are shown in Tables 1, 2 and 3.
Properties of SB microwave pyrolytic oil
| Properties | Pyrolytic oil |
|---|---|
| Appearance | Dark brownish |
| Moisture content (wt%) | 56.3 |
| Total ash (wt%) | 60.19 |
| Density (g cm−3) | 2 |
| Total acid (mg KOH g−1) | 168 |
| pH | 3 |
| CV (MJ kg−1) | 21.41 |
Functional groups and classification of compounds detected in the SB microwave pyrolytic oil
| Frequency range (cm−1) | Functional groups | Classification of compounds |
|---|---|---|
| 3600–3300 | O–H stretching | Alcohol |
| 3050–2800 | C–H stretching | Alkanes |
| 1750–1650 | C=O stretching | Ketones, aldehydes and carboxylic acid |
| 1650–1580 | C=C stretching | Alkenes |
| 1550–1490 | NO2 stretching | Nitrogeneous compound |
| 1470–1350 | C–O stretching | Alkanes |
| 1300–950 | O–H stretching | Primary, secondary and tertiary alcohols, phenols,esters, ethers |
| 915–650 | C–H deformation | Aromatic compounds |
Chemical composition of SB microwave pyrolytic oil [gas chromatography–mass spectroscopy (GC–MS) analysis]
| Chemical compounds | Chemical formula | Percentage area (%) |
|---|---|---|
| Aldehydes | ||
| 4-Hydroxy-3-methoxybenzaldehyde | C8H8O3 | 0.55 |
| 4-Hydroxy-3,5-dimethoxybenzaldehyde | C9H10O4 | 0 |
| 3,5-Dimethoxy-4-hydroxycinnamaldehyde | C11H12O4 | 1.08 |
| 3-(4-Hydroxy-3-methoxyphenyl)prop-2-enal | C10H10O3 | 0.38 |
| 5-Methyl-2-furancarboxaldehyde | 6.01 | |
| Total | 8.02 | |
| Ketones | ||
| 1-(3-Hydroxy-4-methoxyphenyl)ethanone | C9H10O3 | 0 |
| 1-(4-Hydroxy-3,5-dimethoxyphenyl) ethanone | C10H12O4 | 2.18 |
| 1-(2,6-Dihydroxy-4-methoxyphenyl)-1-butanone | C11H14O4 | 3.55 |
| 4-Biphenylyl ethylketone | C15H14O | 1.90 |
| 2-Hydroxy-3-methyl-2-cyclopentene-1-one | C6H8O2 | 2.7 |
| Acetophenone | C8H8O | 0.37 |
| 2,3-Dihydro-1-indanone | C9H8O | 0.37 |
| 3-Ethyl-2-hydroxycyclopent-2-en-1-one | C10H12O3 | 0 |
| Total | 11.68 | |
| Monoaromatic compounds | ||
| 2-Methylphenol | C7H8O | 5.91 |
| 4-Methylphenol | C7H8O | 7.12 |
| 2-Methoxyphenol | C7H8O2 | 7.42 |
| 2-Methoxy-5-methylphenol | C8H10O2 | 5.00 |
| 4-Ethyl-2-methoxyphenol | C9H12O2 | 2.55 |
| 2,6-Dimethoxyphenol | C8H10O3 | 11.15 |
| 2,4-Di-tert-butylphenol | C14H22O | 0.89 |
| 2,6-Dimethoxy-4-(2-propenyl)-phenol | C11H14O3 | 3.23 |
| 4,4′-Methylethynabis[2,6-dimethoxyphenol] | C17H20O6 | 0.49 |
| 1,2,3-Trimethoxy-5-methyl-benzene | C10H14O3 | 3.34 |
| 1,1′-Biphenyl, 2′,3′,4′-trimethoxy-6-hydroxymethyl- | C16H18O4 | 0.47 |
| 1,2,3-Trimethoxybenzene | C9H12O3 | 4.45 |
| 2,6-Dimethylphenol | C8H10O | 0.60 |
| 2-Ethylphenol | C8H10O | 0.40 |
| 2-Methoxy-4-propylphenol | C10H14O2 | 0.93 |
| 2,4-Dimethylphenol | C8H10O | 2.16 |
| 4-Ethylphenol | C8H10O | 1.11 |
| 2-Methoxy-4-methylphenol | C8H10O2 | 0 |
| 2-Methoxy-4-(2-propenylphenol) | C10H12O2 | 4.45 |
| 2,6-Bis(1,1-dimethylethyl)-4-methylphenol | C15H24O | 0.38 |
| Total | 57.60 | |
| Esters | ||
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 0 |
| 2,4-Hexadienedioic acid, 3-methyl-4-propyl-, dimethyl ester | 0.44 | |
| 9-Octadecenoic acid, methyl ester | 0.46 | |
| 1,2-Benzenedicarboxylic acid, diisooctyl ester | C18H18O2 | 3.12 |
| 11-Methyl-octadecanoate | C19H36O2 | 1.15 |
| Total | 5.17 | |
Esterification of SB Microwave Pyrolytic Oil
Esterification was performed on the pyrolytic oil to improve its physicochemical properties as fuel material. Esterification was performed by reacting pyrolytic oil, 1 ml with ethanol 1 ml in the presence of 20 µl sulphuric acid with equivalent of w/w ratio oil/ethanol (1:1) and 2% of sulphuric acid. The mixture was heated at reflux for 60–120 min. A general factorial design was used to investigate the influence of esterification factors on the EO properties and optimize the esterification process using 23 general factorial design.
The independent variables of this study were Ethanol:Oil (1:1 and 1:2 mol mol−1), temperature (65–70 °C), and reaction time (60 or 120 min). The responses were total acid, total moisture, total ash, CV and ester yield.
Esterified Pyrolytic Oil (EO) Characterization
The moisture content in oil was removed prior to analysis by passing through the oil on anhydrous sodium sulphate in a funnel. The total acid of the EO was determined via titration method, using acetone as the solvent and KOH solution (0.1 M) as the titrant. During the titration, KOH solution was poured into the burette and the initial burette reading was recorded. 70 ml acetone was poured into a conical flask, followed by one drop of EO and three drops of phenolphthalein. The mixture was swirled to mix well. KOH solution was slowly added into the conical flask until a change of colour was observed.
The CV of EO was determined using 6400 Automatic Isoperibol Calorimeter. The oil sample (0.5 g) was placed on a sample pan of the calorimeter which was then connected to the terminal inside the bomb by using 10 cm of nickel ignition wire. The sample was then placed inside the bomb, set up into calorimeter vessel and run for analysis automatically.
The GC–MS analysis was carried out to determine the type of organic compounds presents in the pyrolytic oil and EO. The oil sample (1 µl) was diluted with dichloromethane (999 µl) prior to GC–MS analysis (Model: Perkin Elmer-Clarus 600T). Separation was performed using BPX5 (30 × 250 × 0.25 mm) capillary column and detected using MSD detector. The temperature started at 35 °C and held for 2 min. The heating rate was 20 °C min−1 and final temperature of 250 °C was held for 20 min. The detector and injector port temperature were set at 280 °C (Table 4).
Esterification experimental run with coded value at various levels
| Run | Ethanol: oil (mol mol−1) | Temperature (°C) | Reaction time (min) |
|---|---|---|---|
| 1 | 1:1 | 65 | 120 |
| 2 | 1:1 | 70 | 60 |
| 3 | 1:1 | 65 | 60 |
| 4 | 1:1 | 70 | 120 |
| 5 | 1:2 | 70 | 120 |
| 6 | 1:2 | 65 | 120 |
| 7 | 1:2 | 70 | 60 |
| 8 | 1:2 | 65 | 60 |
Results and Discussion
Esterification of SB Microwave Pyrolytic Oil
Factorial experimental design analysis was used to investigate the effect on the amount of alcohol, temperature and reaction time to the physical and chemical properties of the pyrolytic oil. All eight experiments were carried out based on the 23 factorial experimental designs to generate better pyrolytic oil via esterification process. The result for experimental factor is tabulated in Table 5. Maximum and average prediction variance of the design was 0.875, which indicated that the model prediction can be used to navigate the design. Term A represents ethanol:oil ratio factor, while term B represents temperature factor and C represents reaction time factor. Esterification was performed following the method and parameter by Aziz et al. [7].
Experimental factors and response of total acid, total moisture, total ash calorific value and ester yield
| Run | Term A | Term B | Term C | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 |
|---|---|---|---|---|---|---|---|---|
| EtOH:oil ratio | Temperature (°C) | Reaction time (min) | Total acid (mg KOH−1 g−1) | Total moisture (wt %) | Total ash (wt %) | CV (MJ kg−1) | Ester yield (% area) | |
| EO1 | 1:1 | 70 | 60 | 56.1 | 3.07 | 0.21 | 20.48 | 38.95 |
| EO2 | 2:1 | 70 | 120 | 123.42 | 3.32 | 1.06 | 20.63 | 38.67 |
| EO3 | 2:1 | 70 | 60 | 123.42 | 3.45 | 1.01 | 21.57 | 31.34 |
| EO4 | 1:1 | 65 | 120 | 39.27 | 3.08 | 1.49 | 20.15 | 36.5 |
| EO5 | 1:1 | 70 | 120 | 89.76 | 2.95 | 1.28 | 20.47 | 31.87 |
| EO6 | 1:1 | 65 | 60 | 67.32 | 3.32 | 0.44 | 20.21 | 55.11 |
| EO7 | 2:1 | 65 | 60 | 72.93 | 3.1 | 0.31 | 21.11 | 44.16 |
| EO8 | 2:1 | 65 | 120 | 70.25 | 3.28 | 1.01 | 20.3 | 40.38 |
Esterified oil (EO) of pyrolytic oil showed an increase in acid value when the manipulated variables was EtOH:Oil ratio, temperature and reaction time were fixed. The minimum and maximum acid value is 39.27 mg KOH−1 g−1 and 123.42 mg KOH−1 g−1 respectively. EO3 has the lowest acid value of 39.27 mg KOH−1 g−1. It was observed that as the EtOH:Oil ratio increase, the total acid were in TOs were increased. Total acid in EO1 increased from 67.32 to 72.93 mg KOH−1 g−1 with EtOH:Oil ratio increase from 1:1 to 2:1. Total acid in EO2 increased from 56.1 to 123.42 mg KOH−1 g−1 with EtOH:Oil ratio increase from 1:1 to 2:1 and so on. Therefore, EtOH:Oil ratio contributed to the total acid in the TO, lower fraction gives lower acid number.
The highest moisture content was EO6 as much as 3.45% while the lowest was EO4 as low as 2.95%. The moisture content does not vary significantly among one another. It was observed that there were no obvious pattern of single parameter affecting the total moisture. However, there was a reduction in moisture content when the manipulated variable was reaction time in EO1 and EO2, EO3 and EO4, and, EO6 and EO8. Moisture increased in EO5 and EO6, and, EO7 and EO8, when the manipulated variable was temperature. Opposite changes in moisture was observed in EO1 and EO2, and, EO3 and EO4, showed a decrease in moisture content. Higher EtOH:Oil ratio tend to increase the moisture value in most of EO except in EO1 and EO5 showed a reduction in total moisture.
The lowest and the highest total ash were 1.49% by wt in EO3 and the 0.21% in EO2 respectively. The lowest total ash stand on top 3 have the same reaction time of 60 min and the highest total ash were resulted from 120 min of transesterification reaction time. Shorter reaction time of esterification reducing the total ash of EO. Specifically, each EO with constant ethanol to oil fraction and temperature show the same pattern whereby the total ash showed a reduction from reaction time of 120–60 min. It can be seen from the response that the total ash of EO are greatly influenced by the reaction time.
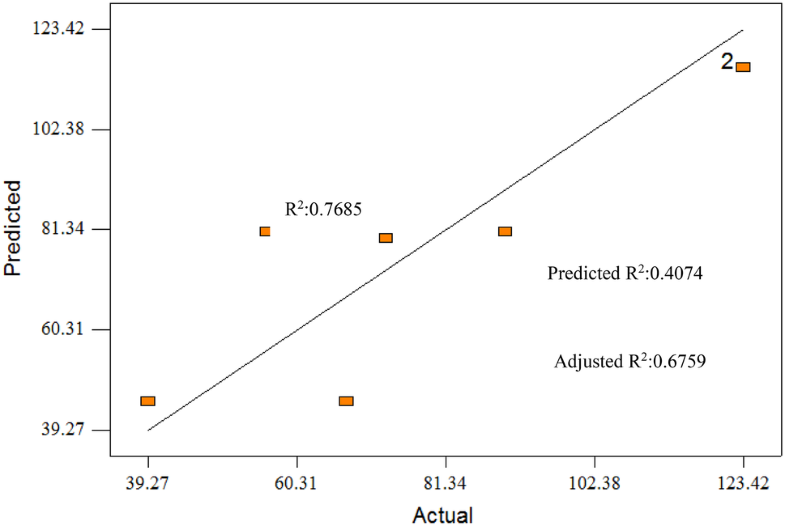
The ANOVA results summary generated by Design Expert shows that the total acid value for EO can be represented by 2 factor interaction (FI), with F-value of 8.30 which implies that the model is significant. Term A and term B are selected, gave values of “Prob > F” = 0.0258, < 0.0500, indicated that the A, B model terms selected are significant. There is likely about 2.58% that the model F-value this large could occur due to noise. The predicted versus actual plot for total acid is shown in Fig. 1. Zero R2 value indicated that there is no relation between the actual and predicted values. Although the predicted R2 of 0.4074 is not as close to adjusted R2 of 0.6759 expected, adequate precision for the model design is > 4, 6.652 predicting responses within the space that experiment explored and model predict the mean well [19]. EO4 showed the lowest total acid produce, 39.27 mg KOH−1 g−1 which was the optimized weight percentage. Term A for this run is 1:1, Term B is 65 °C, Term C is 120 min.
Predicted versus actual plot for total acid (mg KOH−1 g−1)
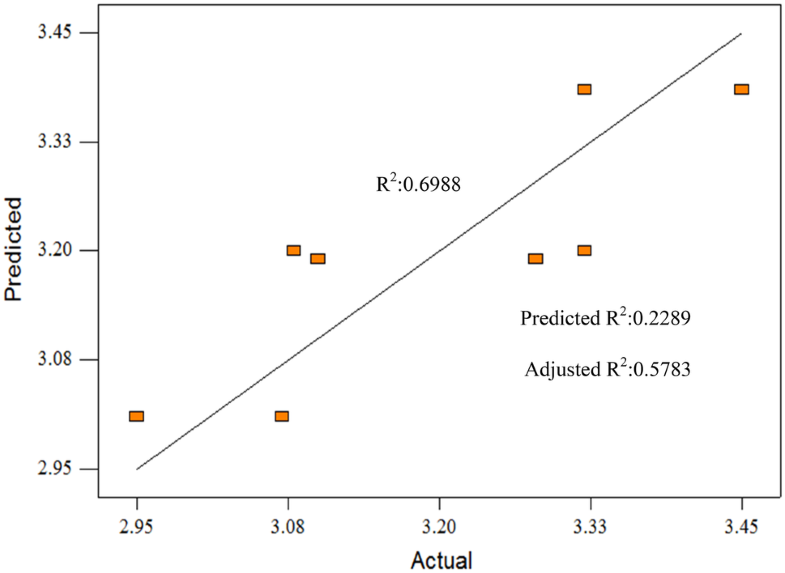
The ANOVA summary for total moisture of EO gave model F-value of 5.80, implies that the model is significant. Term A and term AB are selected, gave values of “Prob > F” = 0.0498, < 0.0500, there are no significant model terms affecting total moisture. The chance that this model could likely occur due to noise is only about 4.98%. R2 is 0.6988, and adjusted R2 is 0.5783. The predicted versus actual plot for total moisture of EO is shown in Fig. 2. Predicted R2 is not as close to the adjusted R2 but Adequate Precision of 5.560 allows the model to be used to navigate the design space. Run 5 showed the lowest total moisture produce, 2.95 wt% which the optimize weight percentage. Term A for this run is 1:1, Term B is 70 °C, Term C is 120 min.
Predicted versus actual plot for total moisture (wt%)
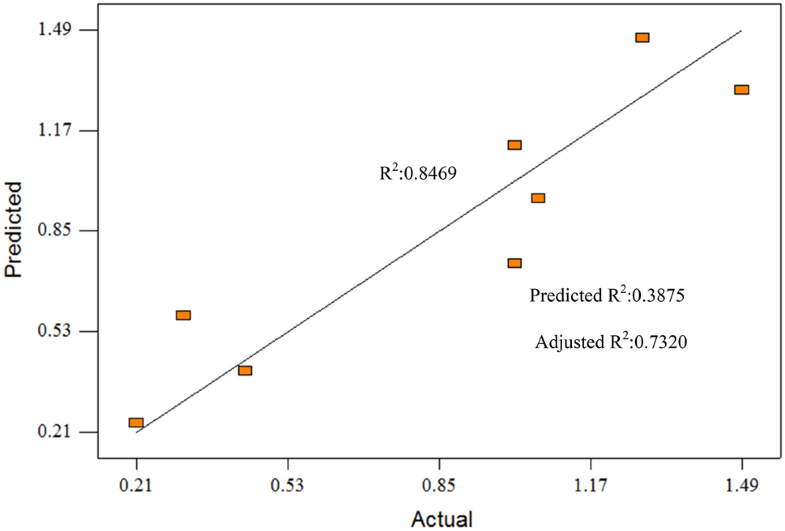
The ANOVA summary of total ash of EO gave Model F-value of 7.37 with only 4.17% chance that the model was affected by noise. Term C, AC, and ABC are significant model terms for total ash. R2 is 0.8469 and adjusted R2 is 0.7320. Figure 3 shows the predicted vs actual plot of total ash in EO. Predicted R2 of 0.3875 is not close to the Adjusted R2. Adequate Precision is 7.106 indicates an adequate signal for the model to navigate the design. Run 1 showed the lowest total ash produce, 0.21 wt% which the optimize weight percentage. Term A for this run is 1:1, Term B is 70 °C, Term C is 60 min.
Predicted versus actual plot for total ash (wt%)
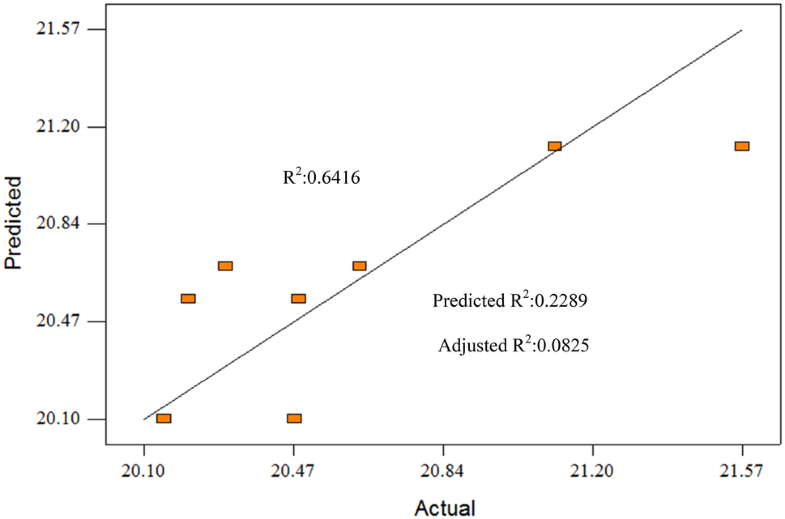
The ANOVA summary for CV gave Model F-value of 4.48, implies that there is 7.69% chance the model F value this large could occur due to noise. Term A and term Care selected, gave values of “Prob > F” = 0.0769, < 0.0500, term C is not significant model terms affecting CV. R2 is 0.6416, and adjusted R2 is 0.4982. Figure 4 shows the predicted vs actual plot of CV in EO. Predicted R2 is not as close to the adjusted R2 but Adequate Precision of 4.863 allows the model to be used to navigate the design space. Run 3 showed the highest CV produce, 21.57 MJ kg−1 which the optimize weight percentage. Term A for this run is 2:1, Term B is 70 °C, Term C is 60 min.
Predicted vs actual plot for CV (MJ kg−1)
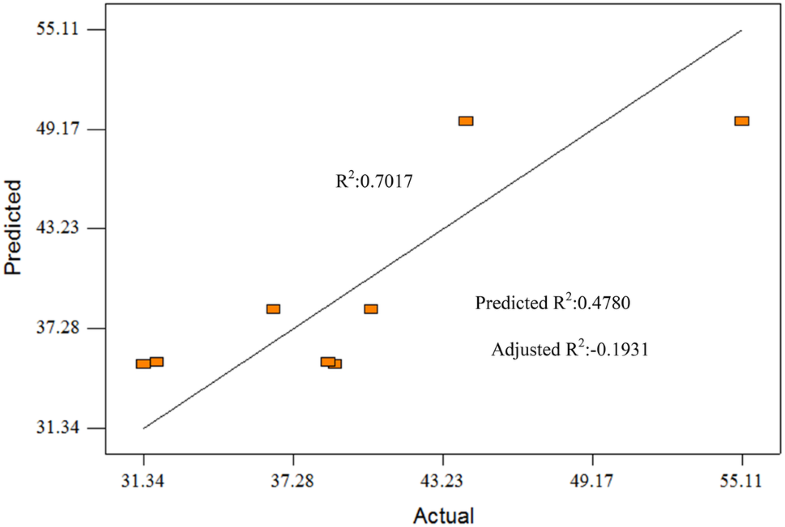
Finally, the ANOVA summary of ester yield gave Model F-value of 3.14 with 14.92% chance that the model occurred due to noise. Term B and C are selected as the factor in the model. In this case, model B is significant model terms. Figure 5 shows the predicted vs actual plot of ester yield in EO. The R2 is 0.7017 and adjusted R2 is 0.4780. Predicted R2 is − 0.1931 far from the Adjusted R2. Run 1 showed the highest ester yield produce, 55.11 wt% which the optimize weight percentage. Term A for this run is 1:1, Term B is 65 °C, Term C is 60 min.
Predicted versus actual plot for total ester (% area)
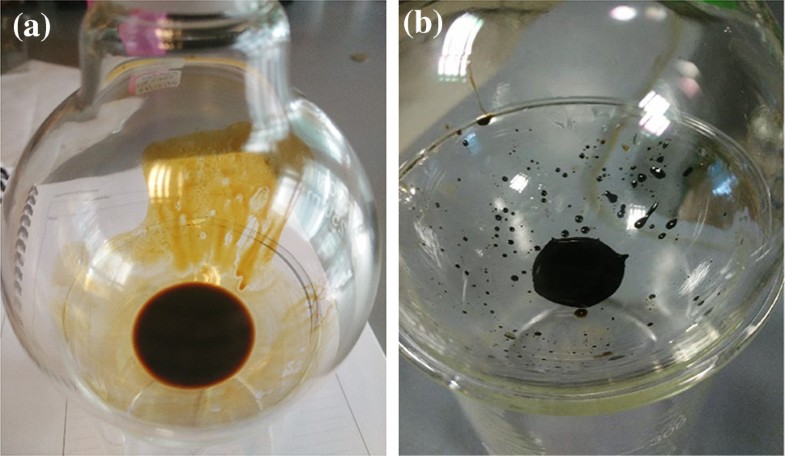
a SB microwave pyrolytic oil, and b EO
The Properties of EO
During esterification process, the pyrolytic oil (Fig. 6a) was transformed into EO (Fig. 6b). EO shows a lot of improvement compared to pyrolytic oil. The moisture content of the EO, 2.95–3.45 wt% was found to be lower compared to pyrolytic oil. Thus, the heating value of EO is improved to be used as fuel. The ash content of EO, 0.31–1.49 wt% was significantly lower as compared to pyrolytic oil, 60.19 wt%. The obvious difference was the colour of the oil, where EO possessed darker colour and less smoky odour compared to the pyrolytic oil. The apparent viscosity (visually observed) of EOs increased, which indicated that the water content was reduced.
EO showed changes from pungent and smoky smell to fruity smell essence and from highly acidic pH 3 to less acidic 4–6. This is in agreement with the previous study whereby the pH value of the EO improved the pH of the pyrolysis palm kernel oil from 3.37 to 5.09–5.12 [7]. Esterification of pyrolytic oil with ethanol accounted for a sharp increase of pH value. The removal of acids decrease the corrosiveness of pyrolytic oil [20]. The physical characteristic of the EO obtained in this study indicates significant changes in density and total acid. Density was decreased from 2 g cm−3 to 0.9–1.1 g cm−3 while the total acid was decreased from 168 to 39.27–123.42 mg KOH−1 g−1. The water content in EO (2.95–3.45 wt%) reduced approximately 52 wt% compared to pyrolytic oil.
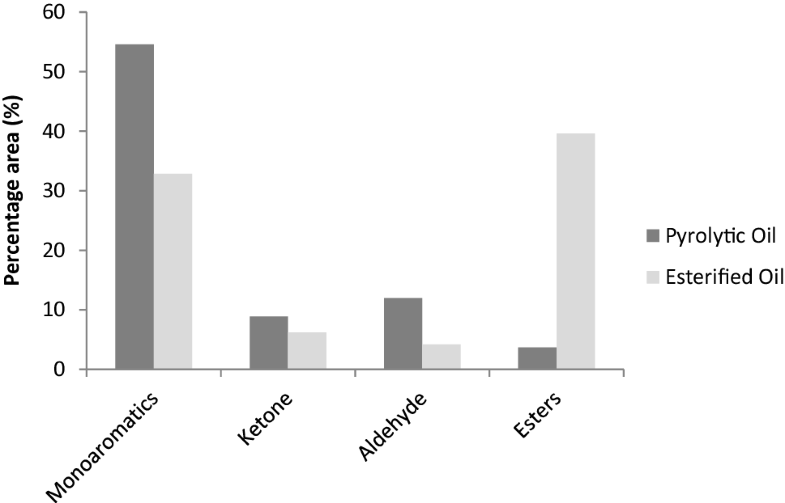
The chemical composition of EO based on GC–MS analysis is shown in Table 6, while the comparison of the average compound in SB microwave pyrolytic oil and EO is shown in Fig. 7. The percentage of esters in pyrolytic oil was increased from 5.17 to 31.34–55.11% after esterification. EO1 gave the highest percentage of ester with 55.11% employing parameters 1:1 (ethanol:oil), 65 wt% of sulphuric acid at 120 °C. Based on GC-MS analysis, the esterification process is successful with ester as a dominated compound followed by monoaromatic compound [20]. However, carboxylic acid compound was not detected in the pyrolytic oil because the type of column chromatography, (BPX5) used was a non polar column. Carboxylic acid is polar compound which cannot be dissolved and eluted in non polar column, BPX5 [21]. During esterification, the carboxyl group presence in the pyrolytic oil is converted into ethyl esters as shown in Scheme 2.
Chemical composition of EO obtained at different operating parameters
| Chemical compounds | Chemical formula | Percentage area (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EO1 | EO2 | EO3 | EO4 | EO5 | EO6 | EO7 | EO8 | ||
| Monoaromatic compound | |||||||||
| 1,2,3-Trimethoxybenzene | C9H12O3 | 0 | 4.18 | 2.71 | 3.52 | 0 | 5.93 | 0 | 3.19 |
| 1,6-Cyclodecanediol | C10H20O2 | 0 | 0.85 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2,5-Dimethoxybenzyl alcohol | C9H12O3 | 0 | 0 | 0 | 0 | 0 | 0 | 4.26 | 0 |
| 2-Methoxy-5-methylphenol | C8H10O2 | 0 | 4.19 | 0 | 0 | 2.26 | 0 | 0 | 4.59 |
| 1,2,3-Trimethoxy-5-methyl-benzene | C10H14O3 | 0 | 3.86 | 3.13 | 4.12 | 0 | 3.35 | 2.39 | 3.79 |
| Desaspidinol | C11H14O4 | 0 | 0 | 0 | 1.62 | 0 | 2.44 | 0 | 0 |
| 2,4-Dimethylphenol | C8H10O | 0 | 0 | 0 | 0 | 0 | 1.59 | 0 | 0 |
| 2,6-Dimethoxyphenol | C8H10O3 | 0 | 7.56 | 6.45 | 6.74 | 2.84 | 0 | 4.26 | 4.43 |
| 2,6-Dimethoxy-4-(2-propenylphenol) | C11H14O3 | 0 | 1.24 | 2.63 | 3.02 | 0 | 4.19 | 0 | 1.57 |
| 2-Methoxyphenol | C7H8O2 | 0 | 6.88 | 6.60 | 7.58 | 2.32 | 6.95 | 4.75 | 6.19 |
| 2-Methoxy-4-methylphenol | C8H10O2 | 0 | 0 | 5.69 | 5.78 | 0 | 4.59 | 2.77 | 0 |
| 2-Methoxy-4-propylphenol | C10H14O2 | 0 | 0 | 0 | 0 | 0 | 0 | 1.59 | 0 |
| 2-Methylphenol | C7H8O | 0 | 6.08 | 1.57 | 5.10 | 0 | 0 | 5.06 | 3.51 |
| 3-Methylphenol | C7H8O | 0 | 0 | 0 | 0 | 3.74 | 0 | 0 | |
| 4,4′-Methylenebis[2,6-dimethoxyphenol] | C17H20O6 | 0 | 0 | 1.94 | 1.68 | 0 | 1.93 | 0 | 1.04 |
| 4-Ethyl-2-methoxyphenol | C9H12O2 | 0 | 2.62 | 2.97 | 3.28 | 0 | 9.11 | 2.23 | 3.10 |
| 4-Methylphenol | C7H8O | 0 | 6.57 | 4.09 | 4.77 | 0 | 5.49 | 4.64 | 5.17 |
| Trans-2,3-Epoxydecane | C10H20O | 5.21 | 0 | 0 | 0 | 3.44 | 0 | 0 | 0 |
| Total | 5.21 | 44.03 | 37.78 | 47.21 | 10.86 | 49.31 | 31.95 | 36.58 | |
| Carboxylic acid | |||||||||
| Cis-9-Hexadecenoic acid | C16H30O2 | 1.97 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclopentanecarboxylic acid, 3,3-dimethyl- | C8H14O2 | 0 | 0 | 0 | 0 | 2.03 | 0.92 | 0 | 0.98 |
| Hexanedioic acid | C6H10O4 | 3.35 | 0 | 0 | 0 | 1.98 | 0 | 0 | 0 |
| Oleic anhydride | C36H66O3 | 1.48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 6.8 | 0 | 0 | 0 | 4.01 | 0.92 | 0 | 0.98 | |
| Ketones | |||||||||
| 1,2-Cyclopentanedione, 3-methyl- | C6H8O2 | 0 | 0 | 0 | 0 | 0 | 0 | 2.48 | 0 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | C6H8O2 | 0 | 2.05 | 2.54 | 4.47 | 0 | 3.56 | 0 | 3.90 |
| 4′-Phenylpropiophenone | C15H14O | 0 | 0 | 2.33 | 2.38 | 0 | 2.42 | 0 | 1.46 |
| 6-Ethyl-3-propionyl-2,3-dihydropyran-2,4-dione | C10H12O4 | 0 | 2.41 | 0 | 2.03 | 0 | 0 | 1.58 | 1.68 |
| Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | C10H12O4 | 0 | 0 | 3.31 | 3.46 | 0 | 3.02 | 1.63 | 2.65 |
| Total | 0.00 | 4.46 | 8.18 | 12.34 | 0.00 | 9.00 | 5.69 | 9.69 | |
| Aldehydes | |||||||||
| Decanal | C10H20O | 8.60 | 1.19 | 1.37 | 2.05 | 5.65 | 2.70 | 2.98 | 2.88 |
| 2-Hexenal, 2-methyl- | C7H12O | 0 | 0 | 0 | 0 | 1.88 | 0 | 0 | 0 |
| 7-Hexadecenal, (Z)- | C16H30O | 0 | 3.44 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | C9H10O4 | 0 | 0.85 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 8.60 | 5.48 | 1.37 | 2.05 | 7.53 | 2.70 | 2.98 | 2.88 | |
| Esters | |||||||||
| Butyl 9-hexadecenoate | C20H38O2 | 0 | 0 | 0 | 0 | 1.19 | 0 | 0 | 0 |
| Cyclohexanol, 2,2-dimethyl-, acetate | C10H18O2 | 0 | 1.93 | 0 | 0 | 0 | 0 | 0 | 2.49 |
| Ethyl oleate | C20H38O2 | 0 | 0 | 0 | 0 | 2.43 | 0 | 0 | 0 |
| Isobutyl 2-methylvalerate | C10H20O2 | 3.61 | 1.19 | 0 | 0 | 0 | 1.58 | 0 | 2.04 |
| Methyl 10-acetoxyoctadecanoate | C21H40O4 | 0 | 0 | 0 | 0.45 | 0 | 0 | 0 | 0 |
| Methyl 13,16-docosadienoate | C23H42O2 | 1.74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Methylnonadecanoate | C20H40O2 | 1.96 | 2.06 | 6.92 | 4.11 | 3.50 | 2.57 | 6.91 | 4.65 |
| 19-Methyl-eicosanoate | C22H44O2 | 0 | 0 | 0.95 | 0 | 0 | 0 | 0 | 0 |
| 21-Methyldocosanoate | C24H48O2 | 0 | 0 | 0.55 | 0 | 0 | 0 | 0 | 0 |
| 12-Oxododecanoic acid, ethyl ester | C14H26O3 | 0 | 4.31 | 5.01 | 6.76 | 3.92 | 4.65 | 5.06 | 9.82 |
| n-Propyl 11-octadecenoate | C21H40O2 | 2.35 | 1.21 | 0 | 0 | 0 | 0 | 0 | 0 |
| n-Propyl 9,12-octadecadienoate | C21H38O2 | 0 | 0 | 2.35 | 1.76 | 1.35 | 1.23 | 1.89 | 1.00 |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 20.51 | 7.62 | 5.84 | 6.01 | 21.78 | 9.96 | 9.18 | 8.87 |
| 11-Octadecenoic acid, methyl ester | C19H36O2 | 0 | 0 | 5.00 | 3.59 | 0 | 2.50 | 4.85 | 2.73 |
| Acetic acid, 13-hydroxy- 4,4,6a,6b,8a,11, 11,14b-octamethyldocosahydropicen-3-yl ester | C32H54O3 | 1.84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzoic acid, 4-hydroxy-, ethyl ester | C9H10O3 | 0 | 0 | 0 | 0 | 0 | 0 | 2.65 | 0 |
| Benzoic acid, ethyl ester | C9H10O2 | 2.12 | 1.59 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzoic acid, methyl ester | C8H8O2 | 0 | 13.68 | 0 | 1.40 | 0 | 0 | 0 | 0 |
| Hexadecanoic acid, ethyl ester | C18H36O2 | 5.24 | 3.40 | 9.88 | 7.79 | 5.51 | 5.60 | 9.84 | 7.07 |
| Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | C35H68O5 | 2.45 | 1.21 | 0 | 0 | 1.95 | 0 | 0 | 0 |
| Palmitic acid vinyl ester | C18H34O2 | 3.35 | 0.75 | 0 | 0 | 1.61 | 0 | 0 | 0 |
| Pentanoic acid, 4-oxo-, ethyl ester | C7H12O3 | 0 | 0 | 0 | 0 | 0 | 3.25 | 0 | 0 |
| Pivalic acid, 2-methylpropyl ester | C9H18O2 | 0 | 0 | 0 | 0 | 0.92 | 0 | 0 | 0 |
| Hexadecanoic acid, (2-pentadecyl-1,3-dioxolan-4-yl)methyl ester | C35H68O4 | 9.94 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 55.11 | 38.95 | 36.5 | 31.87 | 44.16 | 31.34 | 40.38 | 38.67 | |
Comparison of percentage area (%) of chemical compounds in pyrolytic oil and EO
Proposed reaction during esterification of SB microwave pyrolytic oil
Esterified oil also showed a decrease in monoaromatic compound compared to pyrolytic oil. Before esterification process monoaromatic compound is 57.6%. After esterification process the esterified oil increase to 5.21–49.31%. EO1 the highest ester compound contained the least monoaromatic compound, (5.21%). Thus it can be stated that the organic compound in monoaroamtic compound has changed into ester compound [7]. The reaction happen involved carboxylic acids and phenols in monoaromatic compound are converted to corresponding carboxylate and phenoxide ions. Meanwhile, the high molecular fatty acid in pyrolytic oil has been converted to its corresponding ester such as methylnonadecanoate, 19-methyl-eicosanoate, 21-methyldocosanoate and l-(+)-ascorbic acid 2,6-dihexadecanoate. The low molecular fatty acid in esterification was not observed possibly due to a small fraction of the volatile acid in the esterified oil [7]. This indicated that the ester compounds observed after the esterification are dependent on the organic acid in the pyrolytic oil.
The undesirable compound that is mainly responsible for the ageing reaction, instability and corrosiveness such as ketones and aldehyde were reduced in the esterified oil [22]. Before esterification process, the ketone compounds in pyrolytic oil was 11.68% and aldehyde compound is 8.02%. After esterification process, ketone compounds was in range 0–12.34% and aldehyde compound range 2.88–8.6%.
It is envisaged that esterified oil contains heavy oil as the chromatogram showed similarity of functional groups in pyrolytic oil and esterified oil. This is because esterification via distillation could produce two types of upgrading pyrolytic oil, which are light and heavy oil. The chromatogram spectra of the light oil consisted of various ester compounds while the heavy oil was similar with the original pyrolytic oil [7]. It is believed that the esterified oil obtained in this study might consist of a mixture of heavy oil (volatile component) and (nonvolatile component). No separation was performed on the volatile and nonvolatile component during esterification, which therefore resulted in similar chromatogram spectra of pyrolytic oil and esterification oil.
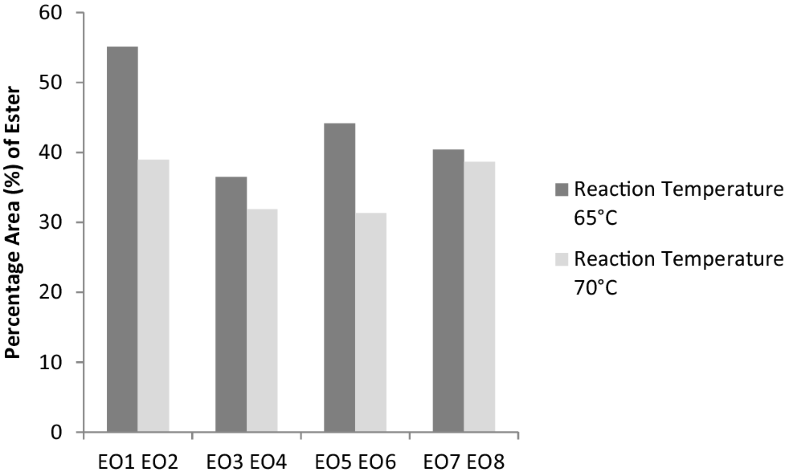
The influence of parameters to ester compounds in esterified oil shows that Term B, temperature and Term C, reaction time play important role to determine the percentage of yield. Esters are the major components presence in the esterified oil. Ester compound in EO1 and EO2 were 55.11% and 38.95% respectively. Figure 8 shows that the formation of ester compounds increased significantly when the reaction temperature was the manipulated variable between EO1 and EO3, and, EO5 and EO7. A decreasing trend in ester compounds was observed in esterified compound when the reaction temperature is increased [23]. As can be seen, with increasing temperature, ester value of the product was found to be decreased, and the least ester value was obtained at EO6, 70 °C and reaction time of 60 min. Similarly, least ester value was obtained at EO4, 70 °C and reaction time of 120 min. The pattern of the graph is supported by [7], ester compounds in temperature 65 °C are higher than temperature 70 °C.
Percentage area (%) of ester at different temperature
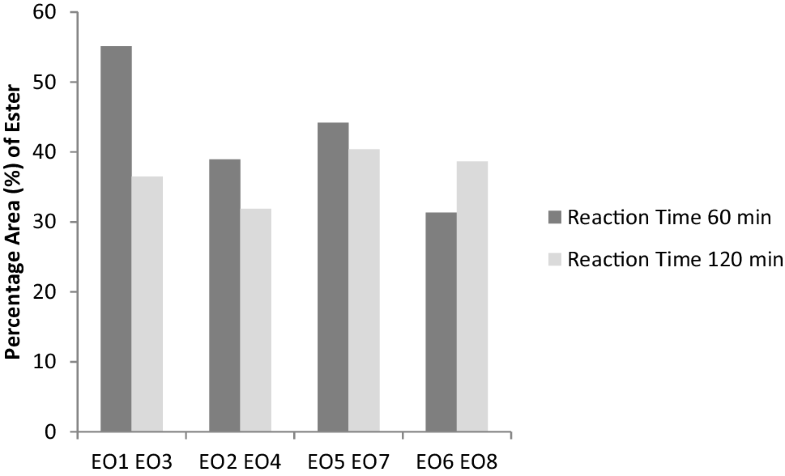
The reaction time (Term C) also shows a similar decreasing trend as in Fig. 9 where high yield of ester was produced at 60 min compared to reaction time of 120 min. This was because the reaction has reached equilibrium hence a reverse reaction starts to occur. Longer time decreases the production yield and this is due to the increase in the probability of EO hydrolysis under acidity condition [24]. Therefore, it is great importance for immediate separation of the produced EO from reaction mixture on getting to the required reaction time. A longer reaction time led to hydrolysis of esters resulted a loss of esters [25]. Although the esters yield of EO8, 120 min was higher than EO6, 60 min when temperature 65 °C, the ethanol ratio to oil is different. The ethanol ratio to oil EO8 is 1:1 while for EO6 is 2:1. Similarly, least ester value was obtained at EO3, 2:1 molar ratio and reaction time of 120 min.
Percentage area (%) of ester at different reaction time
Suitability of EO as Fuel
The EO produced from this study was evaluated for its suitability to be used as a transport grade diesel based on physical characteristics, CV and chemical characteristics. Table 7 shows physical characteristics of EO obtained in this study compared to transport grade diesel obtained from previous studies. Although the CV for EO is considerably of lower quality compared to diesel (42–45 MJ kg−1), but the quality of EO was improved. The lower CV of EO compared to diesel might be due to the existence of oxygenated compounds in the EO. The result is in agreement with the GC–MS analysis whereby high percentage of phenol and its derivatives was observed in the esterified oil. Thus, the EO could possibly be used in the more robust combustion engines, such as slow and medium-speed diesel engines, which require lower acidity and water content than those found in the pyrolytic oil [7]. However, there are more specifications tests needed before the EO can be deemed suitable for real engine applications.
Properties of EO in comparison with SB microwave pyrolytic oil and diesel fuel
| Properties | EO | SB microwave pyrolytic oil | Diesel fuela |
|---|---|---|---|
| Appearance | Black | Dark brownish | – |
| Moisture content (wt%) | 2.95–3.45 | 56.3 | – |
| Ash content (wt%) | 0.31–1.49 | 60.19 | 0.01 |
| Density (g cm−1) | 0.9–1.1 | 2 | 0.82–0.845 |
| Total acid (mg KOH g−1) | 39.27–123.42 | 168 | – |
| pH | 4–6 | 3 | 7 |
| CV (MJ kg−1) | 20.15–21.57 | 21.41 | 42–45 |
Chemical characteristics of esterified oil show that the esterified oil is free of sulphur, low in carboxylic acid and low in oxygen content have beneficial effects in terms of improving the stability of the oil during storage [27]. By upgrading it using the esterification process, formation of acidic tar/sludge and SOx emission can be prevented.
Conclusion
Esterification of pyrolytic oil from SB was performed with ethanol and sulphuric acid (as catalyst) to improve the total moisture, total ash, total acid, density, pH and calorific value of EO. Esterification process has successfully shown improvement in the pyrolytic oil properties with lowest total moisture, total ash and total acid of EO are 2.95 wt%, 0.21 wt% and 39.27 mg KOH g−1. The highest improvement of pH and calorific are pH 6 and 21.57 MJ kg−1. From this analysis, the ethanol to oil fractions show a significant desire changes in total moisture, reaction time shown a significant desire changes in total ash and lastly, temperature had shown a significant desire changes in total acid of esterification process. Esterification at 65 °C for 60 min with 1:1 fraction of ethanol to oil is the optimal condition generated general factorial design RSM. Even so, more studies need to be performed on SB biodiesel especially in calorific value for stabilization and upgrading modifications in parameters and equipment configuration for a better quality of biodiesel.
Notes
Acknowledgements
The authors wish to express their appreciation to the Ministry of Higher Education Malaysia and Universiti Malaysia Sarawak for the financial support [Grant No: PRGS/TK04(01)/1268/2015(02)].
References
- 1.Huang, S., Chiueh, L.: Microwave co-pyrolysis of sewage sludge and rice straw. Energy 87, 638–644 (2015)CrossRefGoogle Scholar
- 2.Tanneru, S.K., Parapati, D.R., Steele, P.H.: Pretreatment of bio-oil followed by upgrading via esterification to boiler fuel. Energy 73, 214–220 (2014)CrossRefGoogle Scholar
- 3.Zhang, L., Yin, M.: Upgrading of bio-oil from biomass fast pyrolysis in China: a review. Renew. Sust. Energy Rev. 24, 66–72 (2013)CrossRefGoogle Scholar
- 4.Prapainainar, C., Yotkamchonkun, C., Panjatharakul, S., Ratana, T., Seeyangnok, S., Narataruksa, P.: Esterification of acetic acid via semi-batch reactive distillation for pyrolysis oil upgrading: Experimental approach. Energy Proc. 52, 559–566 (2014)CrossRefGoogle Scholar
- 5.Fele Žilnik, L., Jazbinšek, A.: Recovery of renewable phenolic fraction from pyrolysis oil. Sep. Purif. Technol. 86, 157–170 (2012)CrossRefGoogle Scholar
- 6.Zhang, Q., Zhang, L., Wang, T., Xu, Y., Zhang, Q., Ma, L., He, M., Li, K.: Upgrading of bio-oil by removing carboxylic acids in supercritical ethanol. Energy Proc. 61, 1033–1036 (2014)CrossRefGoogle Scholar
- 7.Aziz, S.M.A., Wahi, R., Ngaini, Z., Hamdan, S., Yahaya, S.A.: Esterification of microwave pyrolytic oil from palm oil kernel shell. J. Chem. (2017). https://doi.org/10.1155/2017/8359238 Google Scholar
- 8.Gollakota, A.R.K., Reddy, M., Subramanyam, M.D., Kishore, N.: A review on the upgradation techniques of pyrolysis oil. Renew. Sust. Energ. Rev. 58, 1543–1568 (2016)CrossRefGoogle Scholar
- 9.Saber, M., Nakhshiniev, B., Yoshikawa, K.: A review of production and upgrading of algal bio-oil. Renew. Sust. Energ. Rev. 58, 918–930 (2016)CrossRefGoogle Scholar
- 10.Singh, S., Singh, D.: Biodiesel production through the use of different sources and chracterization of oils and their esters as the subtitute of diesel: a review. Renew. Sust. Energ. Rev. 14, 200–216 (2010)CrossRefGoogle Scholar
- 11.Jermolovicius, L.A., Cantagesso, L.C.M., do Nascimento, R.B., de Castro, E.R., Eduardo, E.V., Senise, J.T.: Microwave fast-tracking biodiesel production. Chem. Eng. Process. 122, 380–388 (2016)CrossRefGoogle Scholar
- 12.Bridgwater, A.V.: Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenerg. 38, 68–94 (2012). https://doi.org/10.1016/j.biombioe.2011.01.048 CrossRefGoogle Scholar
- 13.Xiu, S., Shahbazi, A.: Bio-oil production and upgrading research: a review. Renew. Sust. Energ. Rev. 16(7), 4406–4414 (2012)CrossRefGoogle Scholar
- 14.Reyhanitash, E., Tymchyshyn, M., Yuan, Z., Albion, K., Van Rossum, G., Xu, C.: Hydrotreatment of fast pyrolysis oil: effects of esterification pre-treatment of the oil using alcohol at a small loading. Fuel 179, 45–51 (2016)CrossRefGoogle Scholar
- 15.Yu, F., Deng, S., Chen, P., Liu, Y., Wan, Y., Olson, A., Kittleson, D., Ruan, R.: Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover. Appl. Biochem. Biotechnol. 137–140(1–12), 957–970 (2007)Google Scholar
- 16.Ali, E.N., Tay, C.I.: Characterization of biodiesel produced from palm oil via base catalyzed transesterification. Proc. Eng. 53, 7–12 (2013)CrossRefGoogle Scholar
- 17.Chen, W., Luo, Z., Yu, C., Li, G., Yang, Y., Zhang, H.: Upgrading of bio-oil in supercritical ethanol: catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluid. 95, 387–393 (2014)CrossRefGoogle Scholar
- 18.Ghodke, P., Ganesh, A., Mahajani, S.: Stabilization of fast pyrolysis oil derived from wood through esterification. Int. J. Chem. Reactor Eng. 13(3), 323–334 (2015)CrossRefGoogle Scholar
- 19.Osman, M., Azizli, K., Hasnain, M., Bashir, M.J.K. Application of CCD in RSM to obtain optimize treatment of POME using Fenton oxidation process. J. Water Proc. Eng. 8, e7–e16 (2015)CrossRefGoogle Scholar
- 20.Zhang, B., Zhong, Z., Chen, P., Ruan, R.: Microwave-assisted catalytic fast pyrolysis of biomass for bio-oil production using chemical vapor deposition modified HZSM-5 catalyst. Bioresour. Technol. 197, 79–84 (2015)CrossRefGoogle Scholar
- 21.Brown, R.C., Wang, K.: Prospects for fast pyrolysis of biomass. In: Wang, R.C., Wang, K. (eds.) Fast Pyrolysis of Biomass: Advances in Science and Technology, pp. 1–11. RSC Publishing, Cmabridge (2017)CrossRefGoogle Scholar
- 22.Hu, X., Gunawan, R., Mourant, D., Wang, Y., Lievens, C., Chaiwat, W., Wu, L., Li, C.Z.: Esterification of bio-oil from mallee (Eucalyptus loxophleba ssp. gratiae) leaves with a solid acid catalyst: conversion of the cyclic ether and terpenoids into hydrocarbons. Bioresour. Technol. 123, 249–255 (2012)CrossRefGoogle Scholar
- 23.Saha, R., Goud, V.V.: Ultrasound assisted transesterification of high free fatty acids karanja oil using heterogeneous base catalysts. Biomass Convers. Bior. 5(2), 195–207 (2014)CrossRefGoogle Scholar
- 24.Abba, E.C., Nwakuba, N.R., Obasi, S.N., Enem, J.I.: Effect of reaction time on the yield of biodiesel from neem seed oil. Am. J. Energ. Sci. 4(2), 5–9 (2017)Google Scholar
- 25.Eevera, T., Rajendran, S., Saradha, S.: Biodiesel production process optimization and characterizationto access the suitability of the product for varied environmental conditions. Renew. Energ. 34(3), 762–765 (2009)CrossRefGoogle Scholar
- 26.Sharma, B.K., Moser, B.R., Vermillion, K.E., Doll, K.M., Rajagopalan, N.: Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process Technol 122, 79–90 (2014)CrossRefGoogle Scholar
- 27.Lam, S.S., Mahari, W., Cheng, W.A., Omar, C.K., Chong, R., C. T., Chase, H.A.: Recovery of diesel-like fuel from waste palm oil by pyrolysis using a microwave heated bed of activated carbon. Energy 115, 791–799 (2016)CrossRefGoogle Scholar